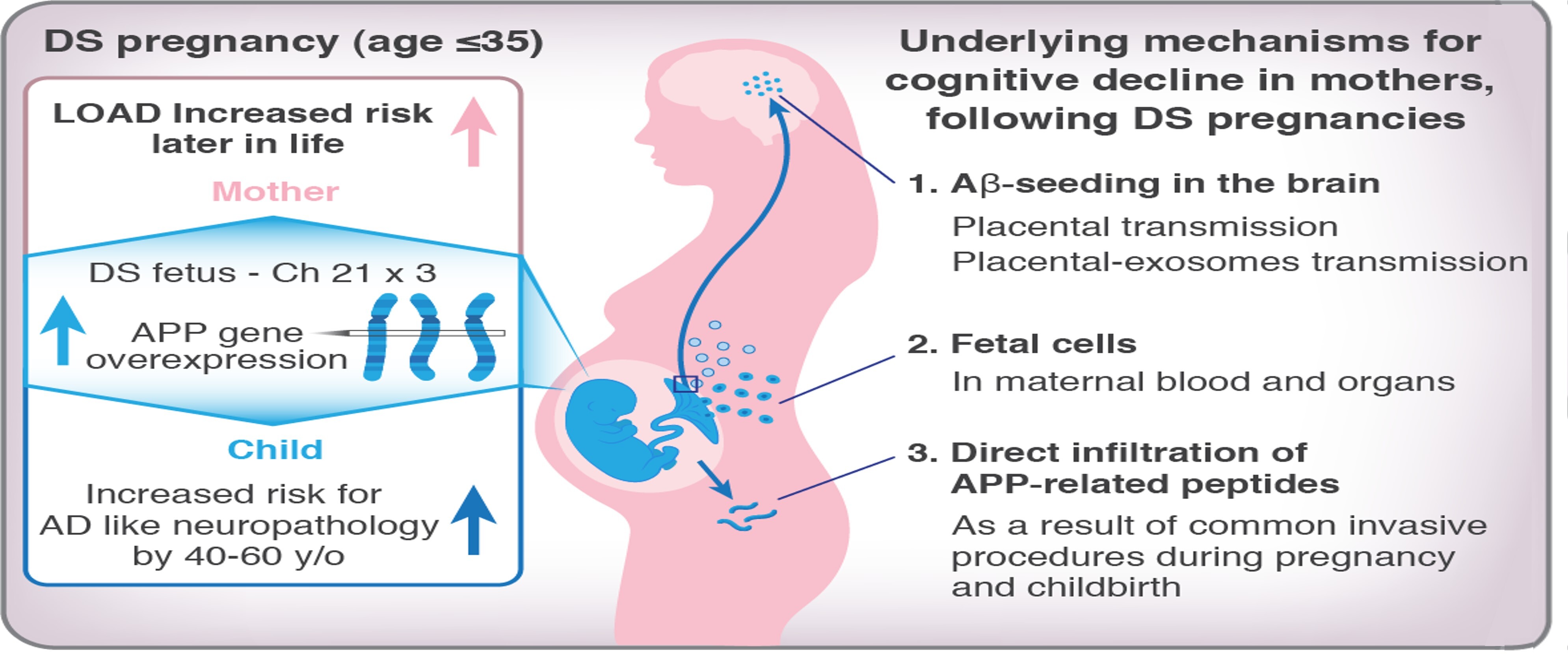
Down Syndrome (DS), which results from a trisomy of chromosome 21 (Hsa21), is the most prevalent genetic cause of intellectual disability worldwide. Some Hsa21 genes are mechanistically involved in Alzheimer's disease (AD) pathogenesis, including the amyloid precursor protein (APP), which is overexpressed in DS individuals, causing early-onset AD-related neuropathology and the Dual- specificity tyrosine phosphorylation-regulated kinase (DYRK) gene, involved in Tau
hyperphosphorylation. This neuropathology includes the accumulation of Amyloid-β (Aβ) deposits and intracellular tangles of hyper-phosphorylated tau protein as early as in the 30’s and 40’s of these patients, respectively, resulting in cognitive decline. Furthermore, an alarming association was found between pregnancy with a DS- affected fetus and a 5-fold increased risk of mothers developing late- onset AD (LOAD) later in life, compared with pregnancies with fetuses affected by other forms of intellectual disability. However, the potential causative link between pregnancy with a DS fetus and maternal LOAD is yet to be established. In the lab, we study whether feto-maternal transfer of APP and its proteolytic fragments occurs during pregnancy with a DS fetus and whether these fetal factors infiltrate the maternal central nervous system, contributing to Aβ-seeding in the mothers' brains, which can further develop into Aβ deposits, affecting mothers' cognitive abilities. Using genomic, transcriptomic, proteomic, and behavioral-cognitive assays, we are studying how fetal APP-related pathology affects mothers that carry hAPP overexpressing fetuses, as in DS, while elucidating fetal APP-maternal transfer properties and the effects of such transfer on maternal cognition to find effective preventative strategies for inhibiting the impact of toxic fetal APP on maternal health.