Optical vs. Action Absorption Spectroscopy
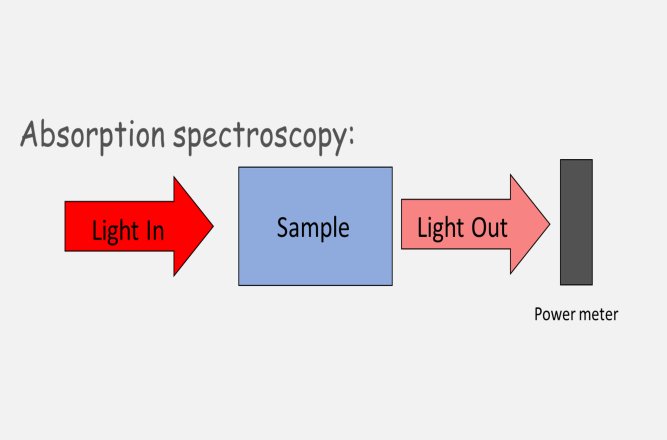
In regular spectroscopy we study a sample by its interaction with light. For example in absorption spectroscopy we shine light through a sample and observe how much of the light does not pass through, how much of it is absorbed.
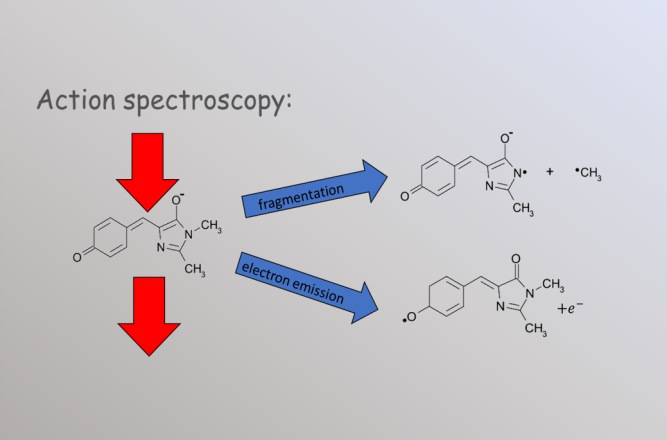
In action spectroscopy we detect photon absoprtion through changes that the photon absorption induces in the traget. For example, if photon absorption causes the ion to fragment or to emit an electron then the resulting fragments can be detected with very high efficiency.
Besides the ability to measure dilute samples, the advantage of action spectroscopy is that we gain a great deal of additional information. For example in the case of fragmentation we can measure the mass of the charged fragment and thus determine the fragmentation mechanism. In electron emission we can measure the velocity and angular disribution of the outgoing electron and thus study the orbital from which it was detached. In both cases we can measure the time between photon absorption and dissociation, and that offers us insight into the thermodynamic properties of even very small molecules. This extra information allows us to study the dynamics of isolated molecules.
