We discovered that a receptor called Par2 (Protease-Activated Receptor 2) is a key mediator of both inflammation and regeneration, capable of promoting either process. Typically, these processes go hand-in-hand, as inflammation is a fundamental step in the body’s recovery from injury. However, in autoimmune conditions, inflammatory responses not only obstruct healing — they actively aggravate injury. In such cases, the immune system mistakenly attacks healthy tissues.
Over the years, studies have shown that Par2 can have completely opposite effects in different damage models. In some situations, Par2 promotes healing and regeneration. In others, it exacerbates inflammation and accelerates tissue destruction. This paradox puzzled the scientific community for years — until our lab proposed a unifying explanation.
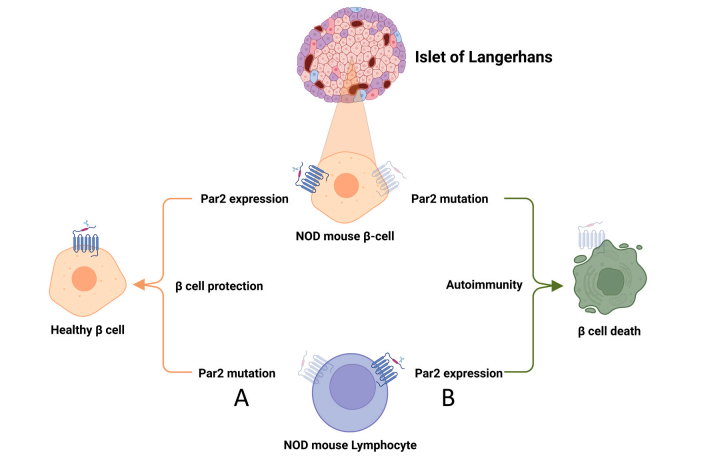
Our research shows that the key variable is location. When Par2 is primarily activated in immune cells, it tends to worsen inflammation and contribute to tissue damage. In contrast, when Par2 is activated in the damaged tissue itself, it can trigger protective and regenerative responses.
To test this theory, we created mice with Par2 selectively removed from specific cell types. For example, in a model of autoimmune diabetes, deleting Par2 from immune cells completely protected the animals from disease. But when we removed Par2 only from insulin-producing β-cells, the disease appeared earlier and more severely. These findings suggest that Par2 can either protect or destroy — depending on where and how it is activated.
We believe this insight has major therapeutic implications. If we can control Par2 signaling in the right tissue at the right time, we may be able to enhance regeneration while preventing immune-mediated damage — a strategy with potential applications far beyond diabetes.